Glen Hodgson, head of Healthcare at GS1 UK, is charged with supporting the NHS and healthcare industry to deliver greater efficiency and a more robust approach to patient safety. He explains more here
A robust supply chain makes all the difference in the world of supply and demand. It is the difference between next day delivery and next week. Take the context of the healthcare supply chain and that is the crucial difference between having access to vital supplies in a timely and efficient manner, and not.
Traceability of healthcare products has become more significant in recent months, not least following the publication of The Independent Medicines and Medical Device Safety (IMMDS) Review, but even more so in light of the Covid-19 pandemic challenging the global healthcare sector. This goes beyond the premise of efficient procurement and inventory management but has a profound impact on patient safety.
GS1’s legacy with enabling traceability
GS1 standards internationally recognised standards used to uniquely identify people, products, and places. For years, their use has been well established in the retail supply chain however, their firm introduction into the healthcare supply chain was first referenced in 2014 in the Department of Health and Social Care’s (DHSC) NHS eProcurement Strategy.
Detailed in the strategy, GS1 standards were proposed to ‘enable trusts to control and manage their non-pay spending, by the adoption of master procurement data, automating the exchange of procurement data and benchmarking their procurement expenditure data against other trusts and healthcare providers’, with a requirement for ‘suppliers to place their product data in a GS1 certified datapool’.
This then became more pertinent when translated into clinical settings with the initiation of the DHSC’s Scan4Safety programme of 2016. Its aims were to ‘free up clinicians to focus on patient care and also support clinicians to provide error-free care, every time’ through the application of global standards.
The impact of traceability in a clinical setting
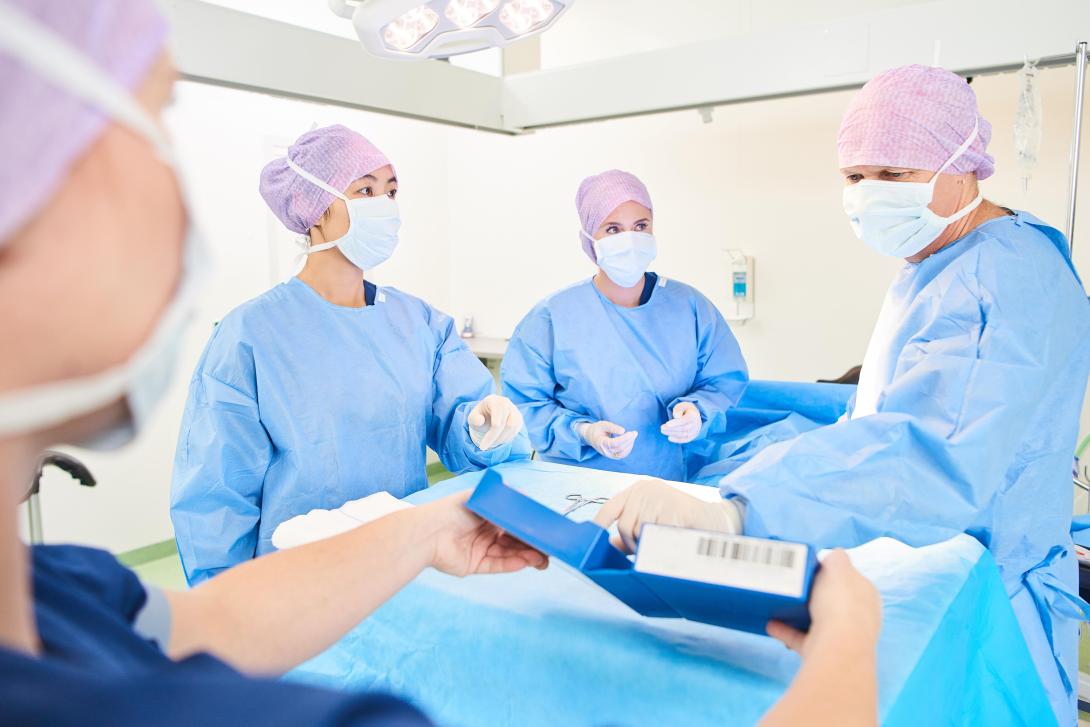
Beyond the Scan4Safety programme, several trusts across the country are implementing GS1 standards to track and trace products. One of the areas where this perhaps has the greatest value is in surgical or invasive procedures.
Take the instance of product recalls at Hull University Teaching Hospitals NHS Trust for an item used in their catherisation labs. Prior to using GS1 standards, the product recall process would likely have taken days to manually locate stock on the premises and notify any affected patients.
However, with point-of-care scanning of the products in place, theatre staff were able to ‘segregate the affected stock within two hours and place a red flag on the system which would alert were that stock scanned at any point in the future’. Within an eight hour period, all consultants that had performed a procedure using the item had been notified and the 60 patients identified as being affected were all notified for follow up.
Beyond Scan4Safety – GS1 standards in the current climate
Today amid the global pandemic, GS1 standards are helping trusts to improve visibility of available stock and enabling trusts to track medical equipment throughout the hospital. The work with University Hospitals Plymouth NHS Trust and their solution provider Paragon ID provides a great case in point for how this has worked in practice.
Using GS1-compliant RFID tags on their medical equipment and Paragon ID’s RFiD Discovery system, the staff and medical equipment teams at Plymouth have the capacity to trace the locations of their labelled assets. Doing so has allowed them to achieve visibility of available equipment stock across the trust, which more importantly serves as an infection prevention control measure. Any equipment returned to medical equipment from a suspected Covid positive ward, can then be contained and dealt with accordingly before being redeployed to another location. It is this level of traceability that we need to aim to achieve across the NHS.
What next for GS1 standards and point-of-care scanning?
Unique identification of products and point-of-care scanning will undoubtedly have a role to play in the future healthcare landscape.
This is perhaps best evidenced by NHS Supply Chain having recently reaffirmed their commitment to the use of GS1 standards for the supply of goods into the NHS. In an August statement released in response to the publication of the Scan4Safety report, NHS Supply Chain announced: “Critical to this is working with our suppliers to ensure that all products are identified with Global Trade Item Numbers (GTINs) which are encoded into GS1 barcodes.”
With the forthcoming Medicines and Medical Device legislation on the horizon, this will become crucially important for suppliers. As of spring next year, suppliers will be required to begin registering their product details for inclusion in what is proposed to be a national perioperative database of procedures using medical devices. Starting with mesh products before expanding to other devices, this comes to bear as a result of one of the nine recommendations made in The IMMDS Review.
The database will enable the Medicines and Healthcare products Regulatory Agency to have a greater oversight of medical device performance for effective post-market surveillance in the event of any adverse event reports or product recall alerts.
Now, more than ever, is a critical time for trusts, manufacturers, suppliers, and solution providers alike to continue driving this traceability work forwards to shape the NHS for years to come. It is the best way to ensure greater patient safety through improved visibility and access to accurate data.
To find out more about GS1 standards in healthcare visit the GS1 UK website.